Abstract
This study explores the application of the CARR Biosystem’s UFMini to achieve concentration and FBS removal from Vero cell cultures using PBS as the wash solution. The successful results demonstrated consistent Vero cell concentration, maintaining high cell viability with minimal loss, while also achieving an 89.6% reduction in FBS levels. The UFMini’s capability to concentrate and wash Vero cells efficiently while preserving their viability in a closed, automated system underscores its potential for optimizing Vero cell culture processes.
Background
Derived in the 1960s from the kidney epithelial cells of African green monkeys, Vero cells have become a valuable asset in vaccine manufacturing, particularly for viral vaccines. Their susceptibility to a wide range of viruses, including those causing polio, measles, mumps, rubella, and influenza, makes them indispensable for viral propagation. Additionally, their ability to support robust viral replication and produce high titers cements Vero cells as the preferred substrate for large-scale viral vaccine manufacturing.
In viral vaccine manufacturing, Vero cells are typically cultured using DMEM-F12, supplemented with 10% FBS. As adherent, anchorage-dependent cells, Vero cells require detachment from the substrate, which is achieved through trypsinization. After detachment, the cells are collected as a suspension using centrifugation or similar methods, and a trypsin inhibitor like FBS is added to prevent further protein digestion and cell damage. Following trypsin neutralization with FBS, the cell suspension is washed using an appropriate buffer like PBS to remove FBS before virus infection. This crucial washing step minimizes potential interference from FBS components and establishes a controlled environment for virus infection and subsequent vaccine manufacturing processing steps, contributing to the production of safe and effective viral vaccines.
The growing demand for an efficient method to remove FBS from Vero cell cultures through PBS washing has become evident in vaccine manufacturing. In response to this need, the CARR Biosystem’s UFMini was evaluated as an instrument capable of concentrating Vero cell cultures and removing FBS through PBS washing, in one automated processing step.
Materials
- 1 UniFuge® Mini (UFMini)
- 1 UFMini Single-Use Module
- 1 L of Vero cells (1.1 x 10^6 cells/mL)
- [1x] [.0067M] PBS
- 300 mL FBS
- DMEM-F12 medium with 10% FBS
- Hach2100Q Turbidity meter
- Vi-CELL BLU Analyzer
Experiment 1
- Vero cells were cultivated on microcarriers in DMEM-F12 medium supplemented with 10% FBS.
- Trypsinization was performed using Trypsin-EDTA to detach the Vero cells from the microcarriers.
- To deactivate trypsin activity, 300 mL of DMEM-F12 medium containing 10% FBS was added to the trypsinized cells.
- The Vero cells were diluted with [1x] [.0067M] PBS to a final volume of 3 L, which was divided equally into three separate 1 L containers.
- The CARR Biosystems UFMini was prepared by installing the UFMini single-use module and tube-set.
- The UFMini bowl was prefilled with 270 mL of [1x] [.0067M] PBS.
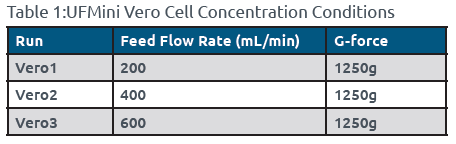
- The Vero cells were fed into the UFMini at a rate of 200 mL/min and subjected to centrifugation at 1250g.
- The Vero cell concentrate was discharged from the UFMini single-use module.
Experiment 2
- The cell concentrate and supernatant containers obtained from UFMini centrifugation runs Vero1, Vero2, and Vero3 were combined, and the cells were resuspended.
- The total combined 3.25 L of Vero cell mixture was spiked with 300 mL (approximately 10%) of FBS, and the mixture was divided equally into three 1 L portions.
- The UFMini bowl was filled with 270 mL of [1x] [.0067M] PBS.
- One liter of Vero/FBS aliquot was fed into the UFMini at a rate of 200 mL/min and centrifuged at 1250g.
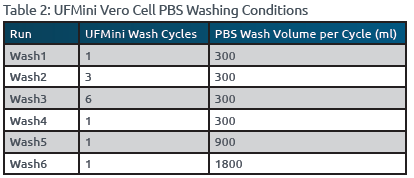
- A single wash cycle of the Vero/FBS aliquot was performed in the UFMini using 300 mL of [1x] [.0067M] PBS.
- The cell concentrate was discharged from the UFMini single-use module.
- Steps 3-6 were repeated twice, but instead using the UFMini washing conditions specified for Wash2 and Wash3, as outlined in Table 2.
- The cell concentrate and supernatant containers from centrifugation/wash runs Wash1, Wash2, and Wash3 were combined, and the cells were resuspended. This mixture was divided equally into three 1 L portions.
- Steps 3-6 are repeated three times, but instead using the UFMini washing conditions specified for Wash4, Wash5, and Wash6, as outlined in Table 2.
Samples of the Vero cell feed, cell concentrate, and supernatant were collected for each UFMini experiment. Cell viability and turbidity of all samples were evaluated using the Beckman Coulter Vi-CELL BLU analyzer and Hach2100Q Turbidity meter, respectively. The reduction of FBS in Wash1-Wash6 was assessed by analyzing the samples for total protein content using the bicinchoninic acid (BCA) assay. The BCA assay results were further validated through SDS-PAGE analysis.
Results
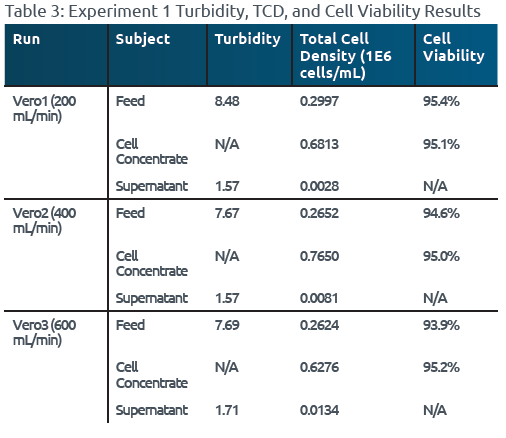
Table 3: Experiment 1 Turbidity, TCD, and Cell Viability Results
Discussion
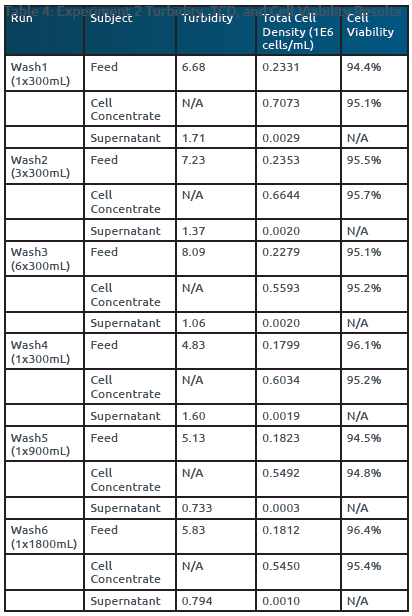
The study involved nine UFMini runs, including Vero1-3 and Wash1-6. The results showed that the Vero cell concentrate samples consistently exhibited higher cell densities compared to the feed samples, indicating successful concentration. Furthermore, the low turbidity values (<2 NTU) in the supernatant across all nine runs indicated efficient collection of Vero cells in the concentrate and minimal cell breakthrough into the supernatant during processing (Reference Tables 3 & 4).
The maximum observed loss of viability was ≤1% across all runs, suggesting that the UFMini effectively cleared cellular debris and maintained Vero cell viability by minimizing shear stress during processing (Reference Tables 3 & 4). This result indicated that the UFMini system was capable of maintaining high cell viability during both concentration and FBS removal through PBS washing.
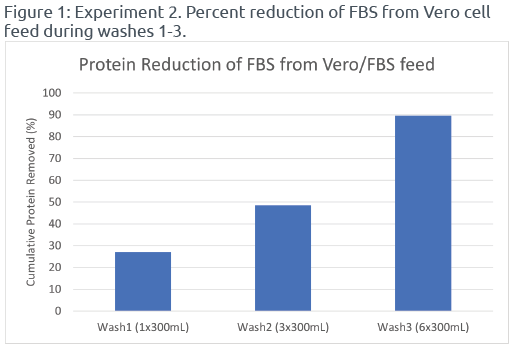
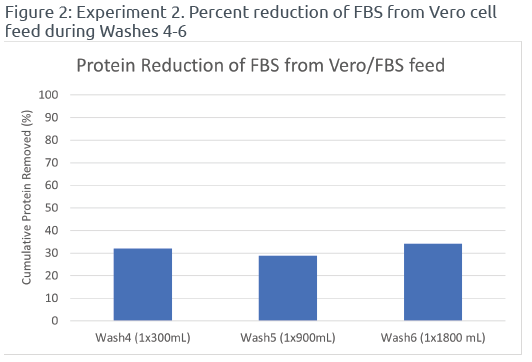
Among the different wash cycles tested, Wash3 (6x300mL) achieved the highest protein reduction percentage of 89.6%, followed by Wash2 (3x300mL) with a reduction of 48.6%. In contrast, single wash cycles (Wash1, Wash4, Wash5, and Wash6) resulted in lower protein reduction percentages ranging from 27.2% to 34.2% (Reference Figures 1 & 2). These findings demonstrated that multiple wash cycles with smaller volumes were more effective in removing FBS from the Vero cell feed compared to single wash cycles. Regardless of the volume of PBS used for washing, single wash cycles exhibited lower efficiency in FBS removal. Therefore, incorporating multiple washes with PBS in the UFMini system, particularly Wash3 (6x300mL), significantly reduced FBS levels in the Vero cell feed.
During centrifugation runs with multiple wash cycles, the UFMini performs a brief period of settling and resuspension before ramping up to separation speed and adding PBS for the next washing cycle. This ensures thorough mixing of the cell suspension with the wash buffer and facilitates efficient removal of FBS and associated proteins from the cells’ surface and the surrounding medium. The process of settling and resuspending the cells in-between wash cycles enhances the overall reduction of protein in the Vero cell feed.
Conclusions
The UFMini system demonstrated effective concentration and PBS washing of Vero cells. The low turbidity values in the supernatant indicated efficient collection of Vero cells in the concentrate with minimal breakthrough into the supernatant. Additionally, the UFMini system maintained high cell viability during both concentration and washing, as evidenced by a maximum observed loss of viability of only 1% across all runs.
Multiple wash cycles proved more efficient in the removal of FBS, with a protein reduction of 89.6% from the Vero cell feed after six wash cycles. The process of settling and resuspending the cells in-between wash cycles enhanced the overall reduction of protein in the Vero cell feed.
Overall, the findings emphasize the UFMini’s potential in optimizing Vero cell culture processes by enabling the efficient concentration and FBS removal through PBS washing, while maintaining cell viability, in a closed and automated system.
The UniFuge single-use family provides low-shear separation, high-recovery performance, and fast processing times in three scalable models: UFMini, UniFuge Pilot, and U2K®. With a variety of bowl sizes and flow rates ranging from 29 ml/min to 20 L/min, the UniFuge family of single-use separation systems offer both scalability and process efficiency in an aseptic closed system.
The UFMini is the smallest scale single-use centrifuge in CARR Biosystems’ UniFuge family. The primary applications for the UFMini are in the harvest and washing of cells for use in cell therapy, cellular agriculture, and cell banking — as well as the clarification of cell cultures during production of Monoclonal Antibodies (mAb) and other therapeutic protein products. The UFMini single-use module provides a closed system for sterile processing and eliminates cross-contamination and the need for CIP and SIP cycles, while reducing change-over time between centrifugation runs.
References
Yilma TD, et al. (2019). Utilization of Vero cell platform for vaccine production. Vaccine, 37(35), 4879-4891. Shahhosseini S, et al. (2020). Vero cells in virus research and vaccine production. Crit Rev Microbiol, 46(2), 138-156.