Sustainable Production
- No flushing cycles, reducing water usage compared to filtration
- Minimized waste from single-use technology

Increase product yield, reduce downstream impurities and cut production costs with the UniFuge® platform. Optimize lentiviral and AAV vector production with low-shear technology for cell separation and overcome titer loss, filter clogging, and contamination in shear-sensitive applications.
Protect sensitive materials and avoid unnecessary filtration steps with UniFuge’s tubular bowl technology. UniFuge offers a low-shear solution for both AAV and lentiviral processing, preventing vector damage and reducing impurities such as HCP contamination in the final gene therapy product.
Connect with an ExpertCell BankingThe closed system harvests and concentrates cells such as HEK, enabling buffer exchange in preparation for cryopreservation. | Gene EditingGentle separation concentrates cells and exchanges media with buffers, preserving cell integrity and enabling vector production. | Seed TrainEfficiently concentrate cells into a high density seed, preparing them for transfer to larger bioreactors for further expansion. |
IntensificationMedia exchange and optional washing maintain cell health for n-1 intensification, enabling advanced manufacturing strategies. | ClarificationLow-shear separation, with optional washing, preserves viral vector integrity and maximizes recovery, ensuring higher yields. | Downstream ProcessingGentle clarification when harvesting whole cells reduces purification burden and enables washing for further viral vector product recovery. |


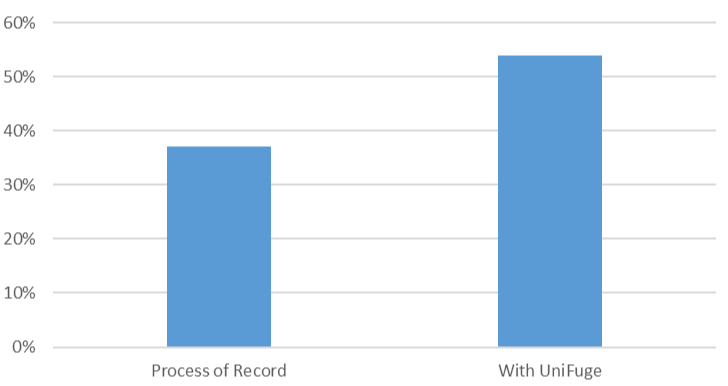
UniFuge overcomes titer loss by minimizing physical damage to viral vectors and cells during clarification and separation steps, increasing vector yield from 37% to 54%, and improving overall yields in gene therapy production.
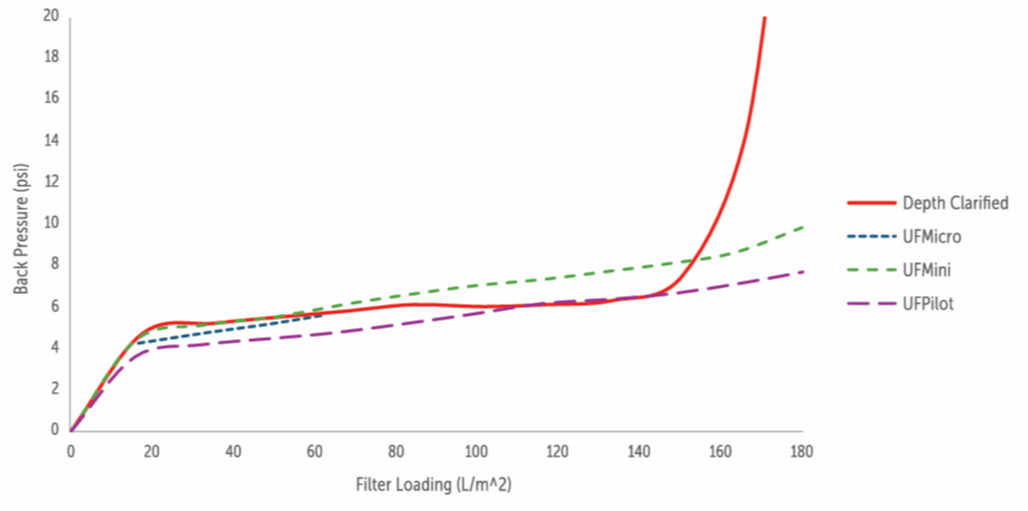
UniFuge demonstrates a reduced need for secondary filtration, with secondary filter loading comparable to primary depth filtration up to 140 L/m2 but with a stable pressure drop when processing tubular bowl broth.
Today’s bioreactors can produce high-density HEK293 cell cultures at volumes of 2000L or more. Traditional depth filtration methods cannot keep pace, resulting in a workflow that is labor and resource-intensive, low titer, and inefficient.
CARR Biosystems partnered with a customer to clarify HEK293 cells containing viral vectors with the UniFuge Mini. Various g-forces (2000–4000xg) and feed flow rates (200–600ml per minute) were tested to find the optimal conditions.
The UniFuge Mini resulted in:
The UniFuge platform uses low-shear tubular bowl centrifugation, which minimizes product loss, reduces HCP contamination and secondary filtration prior to purification. This results in higher product purity, improved yield, and reduced downstream processing requirements.
The UniFuge features a user-friendly design with an easy setup process that takes less than 15 minutes. Its automation capabilities reduce manual intervention and speed up production, saving time and labor.
The UniFuge platform is fully scalable, owing to the family of products that include the Mini, Pilot, and U2k. This seamless scalability allows users to transition from bench-scale processes to large-scale production while maintaining consistency in performance, reducing process variability, and ensuring regulatory compliance.

Get in touch with a sales representative, request support or download our free white paper.
Contact Us