
Enhanced Product Recovery
- Reduced filtration requirements and increased product recovery
- Optimized G-force and low-shear processing preserve viability
- Titer Recovery/grams per measurement
Accelerate mAbs and other biologics development with UniFuge®. Our low-shear centrifuge supports critical processes, including seed banking, cell retention and clarification, enabling process intensification and supporting high product yield and purity throughout development and manufacturing scale-up or scale-out.
Enhance bioprocess efficiency with UniFuge and ViaFuge tubular bowl technology. This low-shear platform automates key production processes in a closed, single-use system, reducing manual intervention and contamination risks. From early stage to GMP production, UniFuge and ViaFuge streamlines workflows, supporting faster, more reliable biologics manufacturing.
Connect with an Expert
Harvest ClarificationEfficient removal of cell debris and impurities to reduce filtration requirements and prepare for downstream processing. |
Cell BankingStandard or high-density, high-volume cell banking with high yield and little to no viability loss. |
Cell RetentionSemicontinuous media exchange in a closed system, retaining cells for further growth and production. |
Inclusion-Body Separation and WashEfficient separation and washing of inclusion bodies, removing impurities and reducing filtration needs. |
Cell Harvest and WashAutomated cell capture and washing for product-containing cells minimizing labor in downstream processing. |
Cell Lysate ClarificationEffective removal of cell lysate debris through high RCF or g-force, reducing or eliminating filtration required. |


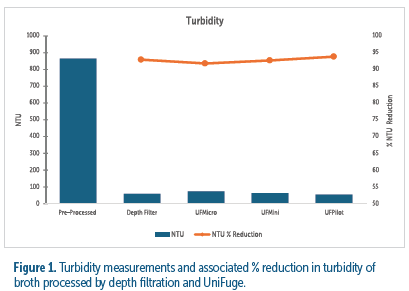
UniFuge models achieve similar turbidity reduction to primary depth filtration, with over 90% reduction, supporting efficient clarification and increasing efficiency for downstream processes.
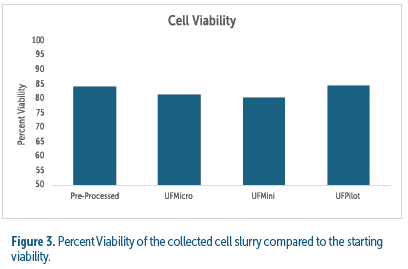
The UniFuge system preserves cell viability across all scales, with percent viability consistently above 80%, maintaining the integrity of cells for high-yield biologics manufacturing.

Biologics, such as vaccines and mAbs, are produced using high-density cell cultures, but traditional primary clarification methods struggle to handle the increased cell mass. Depth filters and disk-stack centrifuges cause shear forces that can damage cells and introduce impurities.
The UniFuge platform was tested for turbidity reduction, cell viability, and lactate dehydrogenase (LDH) levels in CHO cell culture clarification. Flow rates of 100 mL/min, 400 mL/min, and 2000 mL/min were evaluated using the UniFuge Mini, Micro, and Pilot, respectively.
The UniFuge platform delivered:
The UniFuge system, with relative centrifugal force (R.C.F.) up to 4000 xg in its single-use format, efficiently removes cellular debris and particulates from cell culture fluids, allowing secreted proteins and biomolecules to flow through. By automating the harvest step, the UniFuge simplifies the process and reduces or even eliminates the need for complex, multi-stage depth filtration.
The UniFuge system comes pre-sterilized with aseptic connection options for closed-system processing at both small and large scales. Its centrifuge hardware and automation provide streamlined, automated operation. In contrast, most single-use depth filtration pods or cassettes require burdensome setup and sanitization with caustic buffers after installation. They also demand large pre- and post-processing buffer volumes and often result in messy disassembly.
By using the UniFuge for primary clarification, you can significantly reduce or even eliminate the need for depth filtration, along with the associated challenges.
Many single-use bioprocesses that rely on depth filtration generate significant waste due to filter clogging. As more volume is processed, the need for buffers, caustics, and filtration materials increases. In contrast, a single UniFuge centrifuge can process from 10 to over 1000 liters without clogging or needing additional equipment.
Depth filters also require large amounts of buffers and caustics for preparation and decontamination. The UniFuge system comes pre-sterilized with aseptic connections and can be easily drained and disconnected after use, eliminating the need for these chemicals.
Additionally, a large portion of the materials in depth filter pods or cassettes are needed for structural purposes, which increases solid waste. UniFuge modules, however, are designed with durable stainless steel fixtures, allowing a 2 lb device to process up to 1000 liters of cell culture fluid with minimal waste.
Yes, the UniFuge can be used across various stages of the bioprocess workflow, including cell banking, intermittent semicontinuous media exchange (similar to perfusion), cell washing, and buffer exchanges.
The tubular bowl technology utilized in the Unifuge and Viafuge systems offers enhanced titer recovery compared to conventional separation methods like depth filtration. In some instances, these systems can achieve up to 40% higher titer recovery, leading to increased overall product yield. This improvement is particularly beneficial for the production of monoclonal antibodies (mAb) and other biologics, as it supports more efficient recovery and maximizes output.

Get in touch with a sales representative, request support or download our free white paper.
Contact Us