INTRODUCTION
Biologics, such as vaccines, monoclonal antibodies and recombinant proteins continue to grow and advance the promise of life-changing biotherapeutics. Innovation in cell biology and bioprocessing technology have enabled more efficient, intensified processes with higher cell densities. Single-use technologies have enabled flexible facilities with lower manufacturing costs and development timelines that align with clinical speed and rapidly expanding global access. However, intensified processes produce an elevated burden of cell mass on the traditional technologies used in single-use bioprocessing clarification.
Single-use depth filters require large amounts of buffer, time to prepare and dispose of consumables after processing, and are prone to clogging. They can also be a major sources of shear forces that destroy living cells or damage the intended product. Disk-stack centrifuges are effective at removing large volumes of cell material but are known to induce high shear forces, potentially increasing the purification burden of downstream steps. There is also a lack of small-scale models of disk-stack centrifuges, making process development and technology transfer a major challenge.
The UniFuge® cell processing platform by CARR Biosystems™ offers unparalleled scale-down capabilities using tubular-bowl technology to separate cells gently and effectively. This paper will demonstrate how these single-use centrifuges perform through scale-up activities in aspects such as turbidity reduction, shear forces on cells, and minimization of downstream impurities. Additionally, centrifugation as a primary separation approach offers benefits including reduced contamination risks, building space, and buffer requirements due to a reduction in depth filtration. With multiple centrifuge sizes available, processes can be optimized in lab settings and seamlessly scaled through pilot and commercial manufacturing. The results will discuss the potential to improve process efficiency, reduce manufacturing costs, and accelerate process development timelines.
EXPERIMENT
This experiment was prepared to demonstrate scalable parameters for primary clarification of CHO cell culture using the UniFuge platform. Mini® and Micro® single-use centrifugation modules are operated by the UFMini® a benchtop system ideal for small scale and process development work in the 1L to 25L range, and the Pilot® single-use centrifugation modules are operated by the UFPilot, a pilot-scale system for medium production volumes and pilot scale work in the 25L to 500L range. The tested flow rates for the Micro, Mini, and Pilot modules were 100 mL/min, 400 mL/min, and 2000 mL/min, respectively. These flow rates represent reasonable operating parameters for many culture conditions, and may be increased or decreased based on incoming cell culture conditions and process goals. The final test was to perform a two-stage filtration (secondary-depth/ sterilizing) of the resulting clarified CHO supernatant while monitoring back pressure.
Assays performed were cell viability, cell density, turbidity, and a relative lactate dehydrogenase (LDH) assay. These experiments were also performed on the same CHO cell culture clarified using a depth filter at 100 LMH for direct comparability.
RESULTS
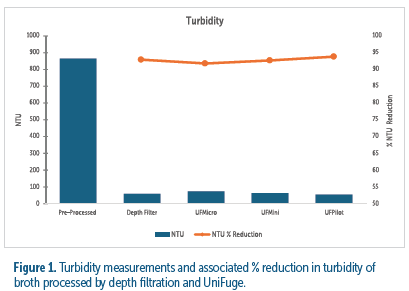
The clarification results from each technology - tubular bowl centrifuge and depth filter - proved to effectively reduce turbidity by greater than 90% to less than 100 NTU (see figure 1). The turbidity of each tested module and corresponding flow rate had an NTU measurement comparable to primary depth filtration, demonstrating comparable performance at each scale.
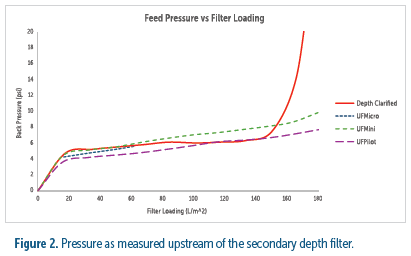
To directly assess the processing impact of utilizing the UniFuge series in place of a primary depth filter, the filterability of broth clarified by each method was compared. These results showed the secondary filter loading from all centrifuges to be comparable to primary depth filtration up to 140 L/m2. However, the secondary filter began to plug rapidly between 140 and 160 L/m2 when processing the depth filter broth and maintained a stable pressure drop when processing the tubular bowl broth (see figure 2). These results indicate that the UniFuge would require less secondary filtration whereas depth filtration would require more to prevent clogging.
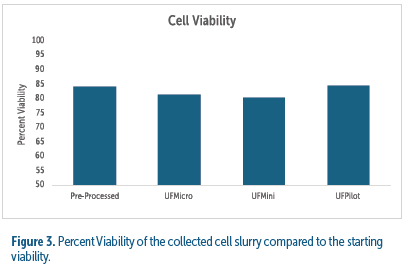
Viability was measured in the cell concentrate (waste stream) as an indication of the level of shearing forces introduced throughout cell processing including pumps, tubing, and single-use bowl assemblies. Viability in slurry from all centrifuge test conditions were within +/- 4.8% of the pre-processed cell culture viability (see figure 3).
Another indicator of cell damage from shear forces is an elevated lactate dehydrogenase (LDH) level. Typically concentrated inside cells, LDH levels in the supernatant can rise rapidly when cell damage occurs during separation. LDH levels were minimally affected by the centrifugation process while depth filter clarified broth had elevated levels, suggesting potential cell damage. This indicates tubular bowl centrifugation can minimize cell debris and host cell protein burden in downstream purification operations.
CONCLUSION
This study has demonstrated how tubular bowl centrifugation performs as a primary clarification technology. At each processing scale, the UniFuge effectively removes cells and cell debris while minimizing additional impurities derived from cell damage. The reduced burden of cell-based impurities entering downstream processing illustrates an advantage of low shear separation.
When designing primary clarification, secondary/polishing, and sterile filtration processes, an important consideration is to balance separation performance and total processing time. These critical process parameters can be adjusted for incoming culture characteristics such as cell type, diameter, density, and viability, as well as overall manufacturing strategies influenced by capacity utilization, operator schedules and consumable logistics. Filtration is scaled by increasing surface area which increases consumable costs and time required to prepare each filter by staging and flushing and to dispose of solid consumable waste. Historically, small scale continuous centrifuges were not available for volumes typically found in development and pilot plants. As shown in this paper, the UniFuge has demonstrated performance and scalable parameters for each size. Therefore, critical process parameters can be tuned to achieve individual process and manufacturing goals throughout scaling activities.
In conclusion, the UniFuge cell processing platform’s robust process parameter range and scalability enable developers to tune manufacturing strategies based on cell culture characteristics, process goals and manufacturing objectives. The inherent separation efficiency of tubular bowl centrifuges and low shear environment enables developers to reduce primary and secondary filtration requirements while balancing processing speed. It not only enhances the manufacturing workflow but also contributes to cost-effectiveness and efficiency.