Introduction
Biotechnology innovation continues to deliver breakthrough life-improving and life-saving medicines around the world. Biologicals, including vaccines, monoclonal antibodies (mAbs) and recombinant proteins, have been produced at large scale for decades with established production methods. However, emerging modalities and next generation biotherapeutics require innovative methods and technologies to support and enable their robust, reproducible, safe, efficient, and economic production at scale.
One such innovation is single-use equipment, which includes a broad range of disposable products employed from upstream and downstream bioprocessing to final formulation and filling. The first single-use bioreactor (the Wave) was introduced in 19961, with traditionally configured stirred tank single-use reactors following in the mid-2000s. Single-use filters were also introduced in the mid-2000s. In 2009, CARR® Centritech (now CARR Biosystems) launched the single-use UniFuge® centrifuge. Single-use equipment has become universally accepted and used for the manufacturing of clinical and licensed drug products.
Single-use centrifuges have the potential to become the standard in bioprocessing due to their advantages versus alternative technologies. For example, high-performance single-use centrifuges rival or exceed the performance of filtration systems in terms of product recovery, cell viability, and purity while also minimizing the risk of contamination. Such advantages directly translate into higher product quality, profitability and, most importantly, better life-enhancing treatments.
Current State of Bioseparation Technologies
Each segment of the biopharmaceutical industry has unique product recovery challenges. However, all segments benefit from technologies that provide lower-shear separation and increase product recovery while minimizing variability and down time. In this paper, we focus on the unique product recovery challenges and opportunities associated with monoclonal antibodies (mAbs), cell therapies, and viral gene therapies.
The first step in the recovery of a biological product is to separate it from the cell culture media, the cell debris, and, in the case of secreted products, the cells themselves. For secreted products, this first step is usually performed by filtration or centrifugation, depending on the volume to be processed and on the product titer.
When processing small volumes (up to 2,000L) or lower titer secreted products, many companies start with depth filtration, as it is relatively easy-to-use, inexpensive, modular and does not require hard piping or special utilities. However, filtration presents challenges in certain situations. First, when volumes scale up, the required storage space becomes impractical, and the material handling and disposal costs become very high. Second, when dealing with higher titer secreted products, there can be significant product losses.
When processing larger volumes (above 2,000L) or higher titer secreted products, centrifugation is the gold standard. Not only is the effectiveness of the separation unmatched, but centrifugation overcomes the challenges posed by filtration at larger volumes and higher titers. The challenge is that stainless-steel centrifuges are large fixed systems, requiring permanent hard-piped installation and resource-intensive cleaning and sterilization between batches.
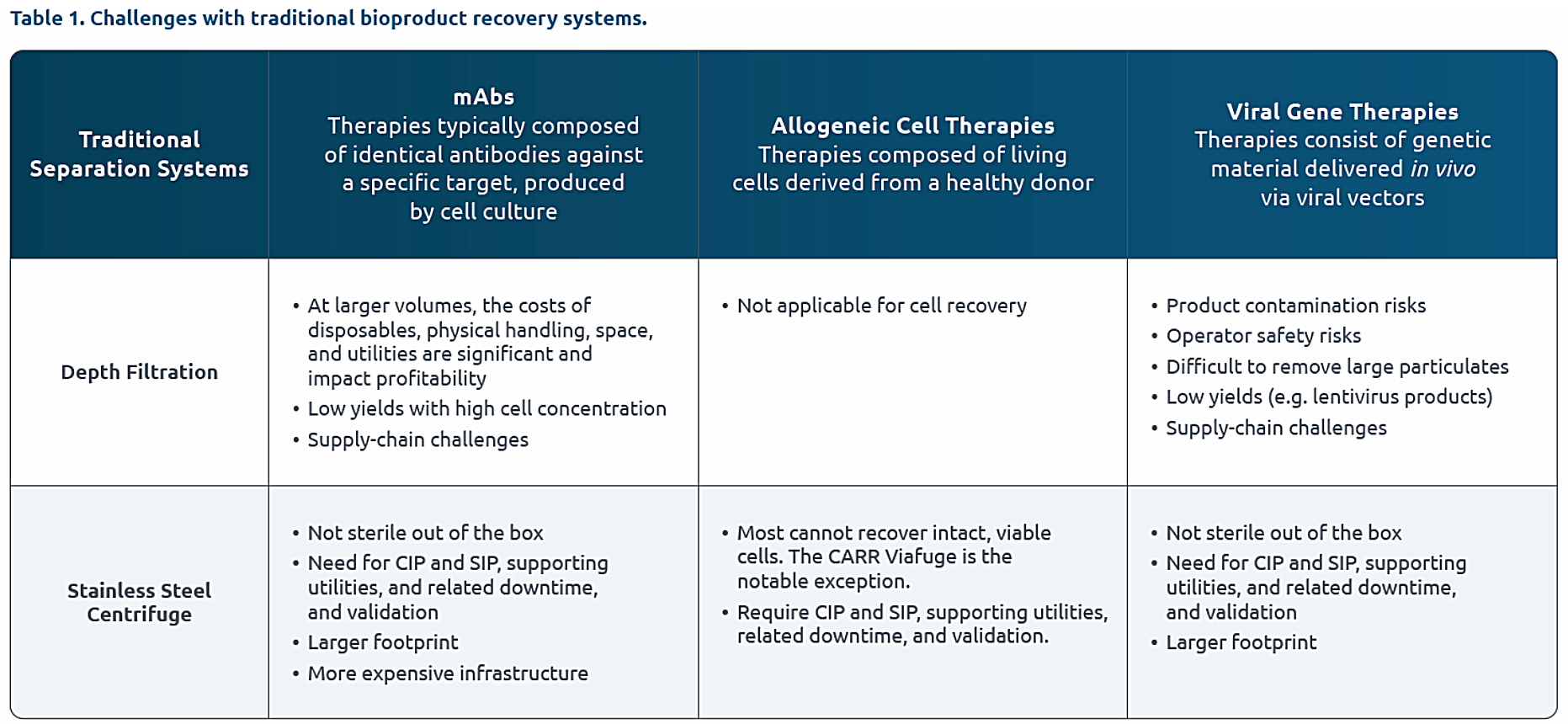
With the challenges of traditional separation technologies, users often must find sub-optimal compromises between performance, practicality, and cost.
Bioprocessing professionals have dreamed about a robust, aseptic, compact separation system that is scalable, does not require special utilities or downtime for extensive cleaning and sterilization between batches, and that enables higher product recovery with excellent quality profiles.
The Case for Single-Use Centrifuges
Single-use centrifuge technologies have emerged as an alternative to primary product recovery processes previously managed by depth filtration and fixed stainless-steel centrifuges. Employing pre-sterilized, gamma-irradiated, disposable modules, single-use centrifuges provide the separation advantages of stainless-steel centrifugation in a smaller footprint, while enabling higher equipment up-time and eliminating the need for special utilities.
Advantages of single-use centrifuges versus filtration and stainless-steel centrifuges include:
- significantly reduced risk to product and operator contamination due to closed system, sterile assemblies and single-use consumables for “out-of-the-box” sterility and plug and play operation;
- streamlined operations and increased equipment up-time, due to elimination of resource-intensive cleaning, validation, and commissioning;
- lower utility and water usage;
- easier mobility and unobtrusive placement within clean rooms due to smaller footprint;
- faster deployment, more streamlined development and tech transfer processes due to the simplicity of installation and operation;
- simplified training and reduced staffing requirement;
- lower cost (on a CAPEX basis when compared to traditional centrifuges and on an OPEX basis when compared to filters) which contribute to increased ROI.
Single-Use Centrifuges in mAb Production
Today, there are over 100 mAbs2 approved by the Food and Drug Administration (FDA) and other agencies. The first monoclonal antibody was approved over 35 years ago and this class of drugs accounts for one-fifth of the FDA’s new drug approvals each year. Bioproduction processes for mAbs are well established and understood. Many long-used blockbuster drugs are manufactured at large scales (greater than 5,000 liters), and in most cases, manufacturers utilize traditional stainless-steel infrastructure with centrifuges for primary product recovery.
However, newer mAbs tend to target smaller patient populations3 and be produced at higher cell density and product titer. Although these smaller production volumes could, in theory, employ depth filtration for primary clarification, the higher cell density and titer are not ideal conditions for filters as they lead to reduced yield and high product losses.4 Whereas titers on legacy products have been below 5 g/L, new products and processes are approaching 10-15 g/L and >50 million cells/mL. When using depth filters in these processes, the yield loss can reach 15-20%. Consequently, the clarification step then becomes a significant challenge for high-density cultures.5
Single-use centrifuges have demonstrated the ability to clarify higher cell concentrations and deliver yields that can reach 98%.6 This capability directly translates to greater efficiency and reductions in the cost of goods sold. A single-use centrifuge investment can pay off with yield gains from a few commercial batches. According to some estimates, using single-use centrifuges may cut down the number of filters used by up to 85%.7 Furthermore, the operating costs of single-use centrifuges are lower, due to reduced storage requirements, savings in buffer volume, and fewer expenses associated with moving and disposing of filters.
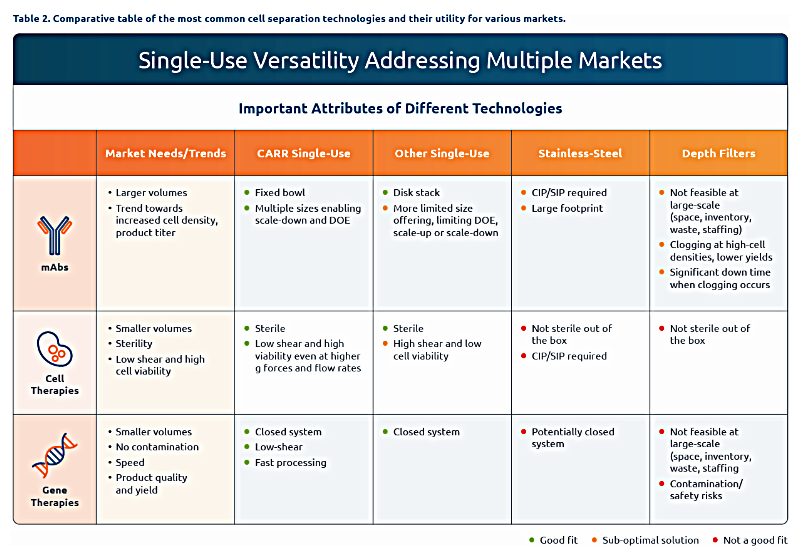 Single-Use Centrifuges in Cell and Gene Therapies Production
Single-Use Centrifuges in Cell and Gene Therapies Production
The field of cell and gene therapies is still emerging and holds enormous potential to revolutionize health care. However, bioproduction processes for these advanced therapy products are not well established and the regulatory pathway is still evolving. Efficient, innovative product recovery solutions are urgently needed to enable safe, reliable, reproducible, and economically viable production processes.
Cell therapies rely on delicate, high value cells to treat patients. Cell viability, cell recovery, and sterility are essential requirements for their production processes. Single-use, low-shear closed-system centrifuges enable high-yield recovery with high viability of cells while ensuring sterility. The UniFuge system produced by CARR Biosystems provides the lowest shear profile in the market, even at higher g forces and flow rates. It also enables flexible cell-washing options that are ideal for high cell viability and high recovery outcomes making it the best separation alternative for cell therapies.
Gene therapies use engineered viral vectors as drug-delivery vehicles that require special handling. Sensitive viral vectors require fast product recovery times, usually under one hour. In addition, large viral vectors fare poorly in filtration as a significant percentage of the product may be retained in the filter resulting in product loss and significant operational challenges. Single-use, closed-system centrifuges significantly reduce contamination risks, reduce processing times, and mitigate the yield losses associated with filtration. All these factors make the efficiency and economic benefits of the UniFuge single-use centrifuges superior alternatives to filtration and stainless-steel centrifugation for viral gene therapy production.
Elevating the Potential of Single-Use Centrifugation
The advantages of single-use centrifuges are generating widespread interest in biomanufacturing. In fact, several licensed products are already being manufactured using UniFuge systems, and many other products are in the clinical or developmental stages. It is important to note that not all single-use centrifuges are equal. Each system presents a different configuration that influences its performance and applicability. The performance characteristics of CARR Biosystems centrifuges present a host of advantages that explain why the UniFuge family is the leader in recovery across multiple modalities:
Low-Shear
The effect and strength of shear stress on cells are key factors for biopharmaceuticals manufacturing. The UniFuge separation systems are specifically designed to minimize damage from shear while effectively separating products. Since cells are not damaged during harvest, the UniFuge reduces cell debris load, improves clarification efficiency, and streamlines downstream processing which equates to decreased cost, faster timelines, and improved product quality. The UniFuge delivers the lowest levels of shear stress in the industry. For cell recovery applications, the yield of viable and undamaged cells is unparalleled.
“Stop & Go” Feature
The UniFuge systems have a functionality to hold material in the bowl/module for washing and buffer exchanges.
Scalability
to commercial manufacturing. Scientists and engineers can progress from the benchtop using the UFMini® to commercial scale processes using the U2k® centrifuge. The UFMini also facilitates process characterization and continuous improvement initiatives as development can be conducted at small scale with direct transfer of operating protocols to clinical and commercial scale manufacturing.
High Product Recovery and Fast Processing Times
The UniFuge allows a diverse range of feed streams and even high cell concentration streams to be efficiently processed. It also allows processing at higher flow rates than other technologies while maintaining a sharp/crisp separation, leading to faster processing times.
Increased Return on Investment
Through higher product recovery, fast processing time, reduced product losses and more streamlined operations, the UniFuge provides an attractive return on investment.
Reliable Supply-Chain
The UniFuge centrifuges and modules are manufactured in multiple sites in the United States to ensure predictable supply. Moreover, CARR Biosystems has offices across the globe to facilitate sales and customer support.
Conclusion
Single-use technologies for biopharmaceutical development and manufacturing are now well-established. They have dramatically improved productivity and capital efficiency and will continue to speed up deployment across clinical and commercial manufacturing sites worldwide.
Single-use centrifugation now allows biopharmaceutical professionals to achieve the benefits of aseptic manufacturing with smaller footprints and streamlined operations that reduce complexity and cost.
the manufacturing suite. The UniFuge centrifuges not only facilitate scale-up, scale-down, and scale-out, they enable linear process development, streamlined tech-transfer, and robust manufacturing for improved drug product quality. In all respects, these advantages contribute to increased return on investment.